
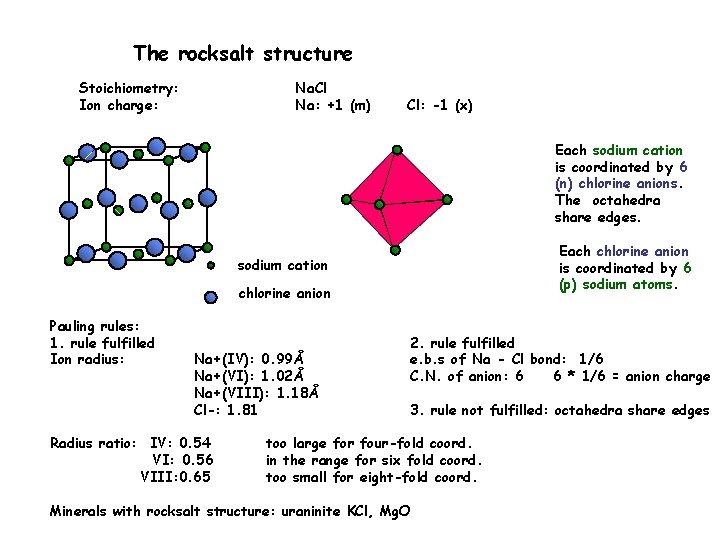
First, although it predicts that the bond angle in H 2 O is less than the tetrahedral angle, it does not make any attempt to predict the magnitude of the decrease. Nevertheless, like any simplified model, it has its limitations. The linearity of the molecule can be appreciated by referring to Figure 9. The molecule is therefore expected to be linear, as is found in practice. Each carbon atom in an acetylene molecule has one bonding pair (to hydrogen) and one superpair (to the other carbon atom). It is less apparent from this analysis, but understandable once it is realized that the superpair is actually two shared pairs ( Figure 9), that the ethylene molecule is predicted to be planar. These three pairs, and the corresponding bonds, adopt a planar triangular arrangement, and the H―C―H and H―C=C angles are predicted to be close to 120°, as is found experimentally. In ethylene each carbon atom possesses two ordinary bonding pairs (linking it to hydrogen atoms) and one superpair (linking it to the other carbon atom). The bond angles in ethane are indeed all close to 109°. In ethane there are four bonding pairs around each carbon atom, so every carbon atom is linked to its four neighbours (one carbon atom and three hydrogen atoms) by a tetrahedral array of bonds. In each case, consider the local environment of each carbon atom.
#CRYSTALMAKER HIGHLIGHT POLYHEDRA WITHOUR CENTRAL ATOM HOW TO#
COVID-19 Portal While this global health crisis continues to evolve, it can be useful to look to past pandemics to better understand how to respond today.
Student Portal Britannica is the ultimate student resource for key school subjects like history, government, literature, and more.Demystified Videos In Demystified, Britannica has all the answers to your burning questions.This Time in History In these videos, find out what happened this month (or any month!) in history.#WTFact Videos In #WTFact Britannica shares some of the most bizarre facts we can find.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.


 0 kommentar(er)
0 kommentar(er)
